Part 1 of this article looked at sensing and sensors for ultracold and cryogenic temperatures; Part 2 advances the story to more esoteric situations.
Q: What’s the fundamental difference between measuring extremely high temperatures versus cryogenic ones?
A: When things get really hot, extending into thousands of degrees, the sensor options are limited. Usually, it becomes a choice between thermocouples of various types or an infrared sensing arrangement. Since the source being measured is at a high temperature, it has a lot of energy which needs to be captured by the sensor with minimal impact on that source – but having all that energy is a key benefit in easing the challenge. Any energy absorbed by the sensor is often negligible compared to the energy of the source itself, and so does not affect the source’s energy and thus temperature.
Q: OK, but what about sensing at the colder end in the low double-digits (a few tens of K), single-digit (1 to 9 K), or even sub-single-digit (<1 K) regions?
A: The low end is now amazingly close to absolute zero. There’s research being done down at 0.01 K, and a recent article in IEEE Spectrum (Reference 3) discussed research being done below 100 nK. (How to get down to that low value is another fascinating story!)
Q: But how do you know exactly where you are when you’re down there?
A: Accurate, credible measurements of these cryogenic temperatures are part of a very strange world, for several reasons:
- First, while the laws of physics are obviously still in force, the materials of the sensors go into major transitions with many of their characteristics and behaviors changing radically. Sensor performance, linearity, and other critical attributes shift dramatically in the low K regions, and these changes can be hard to assess.
- Second, the measurement approach is usually intertwined with methods being used to reach those temperatures. For example, multi-Tesla magnetic fields are often a major part of a supercooling arrangement (again, how and why is another story), and those fields have a major impact on the sensing arrangement and its components.
- Third, projects at deep cryogenic temperatures often involve tiny amounts of mass; in some cases, it can be as little a few atoms or molecules. So, you have a double problem: molecules with low energy, and only a few of them. You obviously can’t attach a sensor, and even if you could, the sensor would seriously affect the substance being measured. There is so little mass and energy in the target being measured doing anything will both distort the target and draw some of its energy. In some ways, it’s a situation analogous to Heisenberg’s Uncertainty Principle of quantum physics, in which the basic act of making a measurement affects that which is being measured.
Q: So what is done to make those measurements?
A: Researcher at these cryogenic temperatures have multiple choices, depending on how low they are going and what they are measuring (solid mass, molecules in a gas-like cluster, or individual molecules). Relatively speaking, those dealing with liquid oxygen (90 K, −183°C) and hydrogen (20 K, −253°C) for rocket fuel have it easy, as do those working with nitrogen (77 K, −196°C). In contrast, liquid helium is much harder to assess, at about 4 K (−269 °C), yet is it used to cool the magnets of MRI machines down to their superconducting regions.
Q: What’s the key to making these cryogenic measurements?
A: Always keep in mind that what we commonly call “temperature” is really a measure of the energy of whatever is being measured. As with nearly all temperature measurements, users must first consider three specifications: what range they need to cover, what absolute accuracy they need (and with what precision (resolution), and the impact their measurement arrangement will have at these temperatures.
Q: Are all cryogenic sensing situations esoteric?
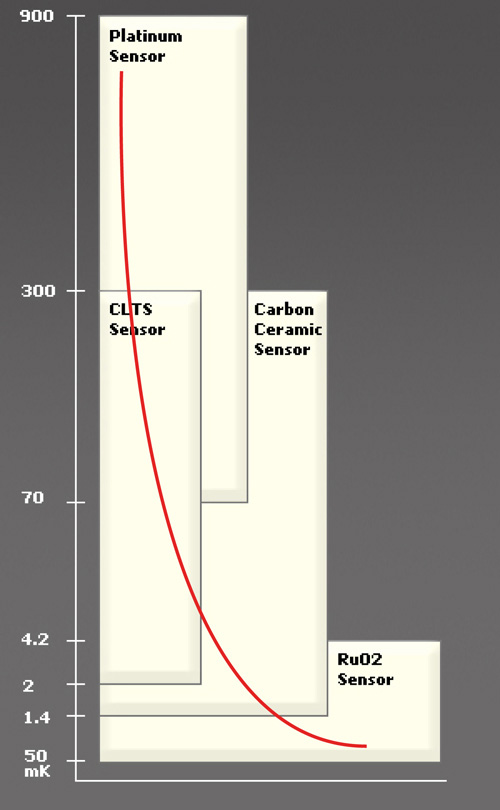
A: No, not at all. Somewhat surprisingly, some sensors which are commonly used at more “normal” temperatures can even work down into the high single-digit region (Figure 1). Among the options are RTDs (using platinum or rhodium-iron), germanium, and even classic carbon-based resistors. However, the intense magnetic fields, which are also a part of many of the cryogenic cooling arrangements, can induce sensor errors of a few K.
Still, the research reality is that there is so much demand for low-K sensing that these transducers are standard, off-the-shelf catalog items available from many vendors (a fact which is fairly amazing when you think about it). Even a “humble” two-plate capacitor can be used in a bridge arrangement with its physical dimensions and shape – and thus its capacitance – changing in a known relationship as a carefully modeled function of temperature. NIST has developed many standard and models using pure materials and alloys, shapes, and configurations, and these can be adopted with care. These, too, are available as standard catalog items such as the CS-501GR (Figure 2), from Lake Shore Cryotronics, Inc. which is specified for about 1.5 K to 300 K (Figure 3).

Q: What are some of the more advanced sensing techniques?
A: More complex options include use of optical techniques such as the use of Brillouin scattering in optical fibers and other sophisticated arrangements. Lasers and laser shifts can be used to provide non-contact measurements as well, but it’s a complicated electro-optical set-up with many sources of inaccuracy requiring calibration, correction, error cancellation, and compensation.
Q: Seems like the problem is pretty much solved, but is it?
A: It may be so for “bulk” materials sensing such as liquid helium, but these techniques will not work for gauging the temperature of small numbers of molecules. In those situations, some truly esoteric approaches are needed. One arrangement sweeps an intense magnetic field with a precision gradient around the trapped target, then observes how its molecules distribute along that field, indicating their energy, and thus, temperature.

Another scheme uses lasers to push the molecules, where the amount of laser energy versus resultant motion indicates the target energy. These and other complex methods are not only difficult to set up, but they require numerous corrections for second and third-order subtleties of their physics, as well as system imperfections.
So, the next time you feel the need to complain about how hard your temperature-measurement scenario is, just think about those working in the low-K region, down to and even below 1 K. It’s a spooky world down there, and any researchers must also ask and answer the eternal instrumentation question, “Even if I am measuring the temperature, how do I calibrate, confirm, and validate my readings?” That’s almost nightmare stuff.
References
- EE World Online, “Magnetic resonance imaging (MRI), Part 1: How it works”
- EE World Online, “MRI, Part 2: Historical development (and lawsuits)”
- IEEE Spectrum, “Quantum Computing: Atomic Clocks Make for Longer-Lasting Qubits“
- Measurement Standards Laboratory of New Zealand, “Kelvin
- National Institute of Standards and Technology (NIST), “The International Temperature Scale of 1990”
- National Institute of Standards and Technology (NIST), “Kelvin: ITS-90”